
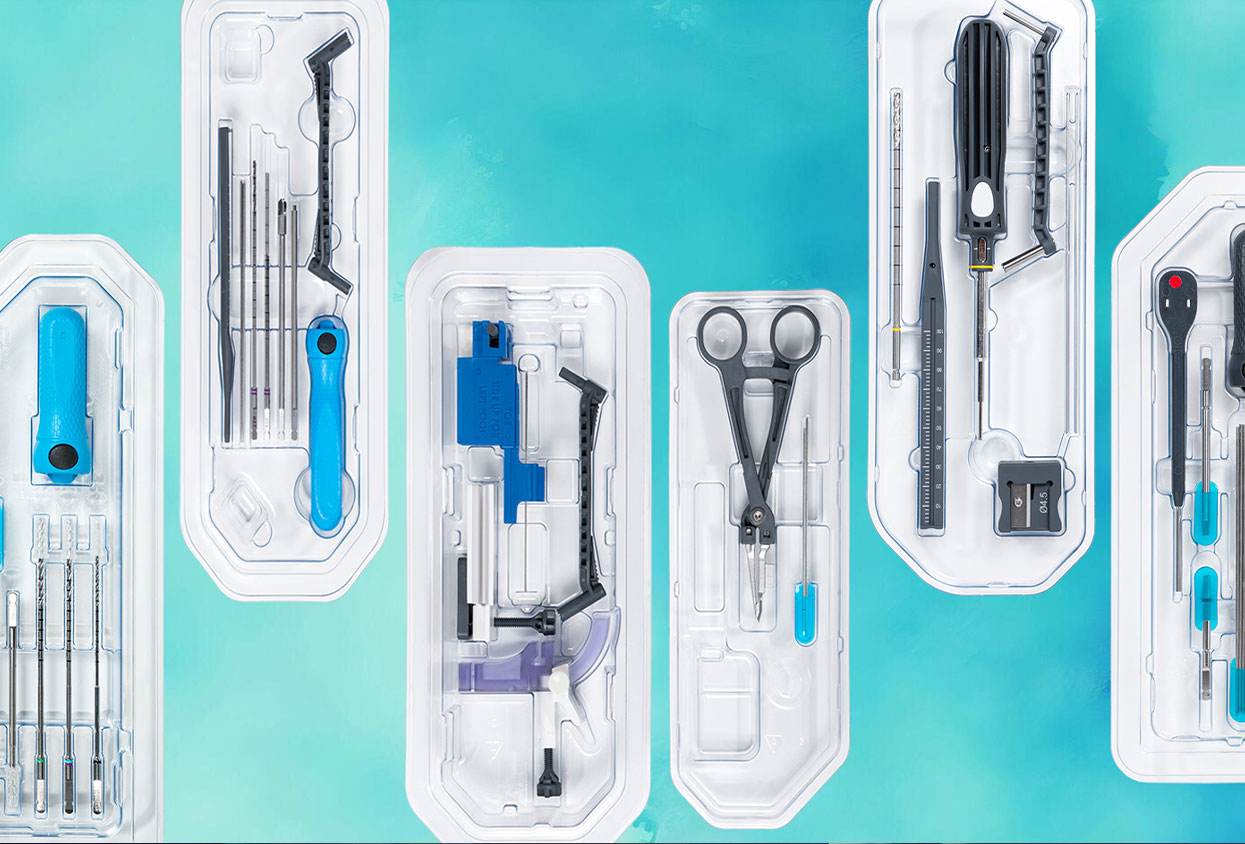
Hospitals and surgery centers around the world now look for smarter, safer ways to care for patients, and that leads them to single-use surgical instruments as part of the answer. What started as a niche solution in a few specialties now sits at the center of conversations about infection prevention, OR efficiency, and consistent clinical outcomes.
At ECA Medical, we see this shift every day as we work with OEM partners, health systems, hospitals and ambulatory surgery centers that want instruments and kits that arrive sterile, ready, and reliable. Surgical teams feel growing pressure from infection risks, reprocessing bottlenecks, staffing shortages, and budget constraints, and they want tools that simplify, not complicate, surgical care.
We call that vision Surgery-Ready™, where Instruments and procedural kits arrive pre-configured and sterile, and they move smoothly into the OR workflow with minimal friction. The result is safer care, less variability, procedural speed and a calmer clinical environment for everyone in the room.
Reusable instruments will always play some role in surgery; however, many hospitals now question whether they should remain the default in high-volume service lines, especially in orthopedics and spine. When leaders look closely at the total impact of reusable sets, several themes repeat.
On the surface, reusable sets can appear economical. A hospital purchases them once and uses them for years. Yet the real cost does not sit in the purchase order; it hides in the day-to-day work of keeping those sets in circulation.
Every reusable set requires:
In high-volume orthopedic and spine programs as well as other procedures, these costs appear on every schedule. As case counts grow, reprocessing teams must handle more complex trays under tighter timelines. The true cost of ownership for reusable systems can easily exceed initial expectations and, over time, rival or exceed a well-designed single-use program.
Reusable instruments follow a long, intricate path. They move from the OR to decontamination, to automated washers, to assembly, to sterilizers, then into storage, and finally back to the OR. Each step must run correctly, with enough time, staff, and oversight.
Even with strong protocols, hospitals still worry about:
Single-use solutions simplify that picture. Instruments and kits arrive factory sterile, sealed in validated packaging, and supported by documented sterilization processes. Staff verify package integrity and shelf life, then bring it into the OR. This straightforward chain of custody reduces variables and provides a consistent sterility profile from one case to the next.
Reusable sets can also create operational friction that affects every patient on the schedule. Missing instruments, damaged tools, and delayed sterilization cycles often lead to:
Every delay affects multiple teams, from pre-op nurses and anesthesia to surgeons and sterile processing. Over a day or a week, that friction adds up and can quietly erode capacity.
Surgery-ready single-use sets remove many of these failure points. Instruments come complete and consistent; they do not leave the OR for reprocessing, and they do not depend on a specific sterilizer cycle finishing on time. This stability allows OR leaders to improve turnover, protect block time, and support more predictable schedules.
The movement toward single-use is not driven by convenience alone. It rests on a commitment to safer, more reliable procedures.
ECA Medical has built its expertise around precision torque limiters and single-use fixation tools that support orthopedic, neuromodulation, cardiovascular and spine procedures. We design these devices to deliver accurate, repeatable torque on every screw, in every case.
Consistent torque helps:
Reusable torque instruments require routine calibration and maintenance. In busy systems, this can become difficult to track perfectly. Single-use torque devices arrive factory calibrated, are used once, and then leave the inventory, so performance stays aligned with design parameters throughout their life.
That consistency gives surgeons and OR teams more confidence, and it supports standardized outcomes across the entire network.
Factory sterile packaging provides a strong foundation for infection prevention strategies. Each instrument and kit passes through validated sterilization with controlled parameters and documented quality controls. Packaging protects sterility during shipment, warehousing, and handling.
For hospitals and ASCs, this approach:
Teams can verify sterility quickly and focus on patient care rather than worrying about how many steps an instrument passed through before reaching the sterile field.
Surgical teams work in a high-stakes, time-sensitive setting. Even highly skilled professionals face cognitive load, interruptions, and shifting priorities during a busy day.
Single-use systems help by:
When instruments arrive procedure-ready, teams can concentrate on clinical steps rather than logistical ones. That shift reduces opportunities for error and supports a safer experience for patients and staff.
Administrators, surgeons, and OR leaders all evaluate the financial and operational effect single-use strategies have on the broader system.
The comparison between reusable and single-use instruments must consider the full lifecycle, not just the sticker price.
Reusable systems incur:
Single-use systems, when properly designed and implemented, eliminate many of these recurring expenses. Hospitals move from complex, variable costs to more transparent per-procedure or per-kit pricing. Staff time that previously went into cleaning and assembly can shift toward higher-value activities, including quality initiatives and direct patient support.
Across a multi-hospital network, this change can unlock significant operational savings and create headroom for reinvestment in clinical programs.
Surgery-Ready™ instruments support faster room turnover and smoother case flow. Staff no longer wait for a specific tray to return from sterilization, nor do they delay while searching for a missing driver or reassembling a complex set.
Benefits include:
As case throughput improves, hospitals can meet demand for high-growth services like joint reconstruction and minimally invasive spine without immediately adding new rooms or major expansions. Patients experience fewer delays and rescheduling events, which strengthens confidence in the organization.
Ambulatory surgery centers operate on leaner footprints, both in space and staffing. Many ASCs do not have large central sterile departments, and they look for solutions that keep their capital and operational models simple.
Single-use instruments and kits fit naturally into this environment:
ECA Medical designs procedure-specific kits that align with ASC goals for efficiency, sustainability, and quality. By reducing equipment complexity, ASCs can focus on what matters most, delivering safe, timely care in a setting that feels organized and controlled.
Any conversation about single-use solutions must address environmental impact. The picture here is more nuanced than it might first appear.
It can be tempting to equate visible solid waste with total environmental impact. A bin of discarded instruments and packaging looks like more waste than a reusable tray returning to decontamination. However, the resource use behind reprocessing is substantial.
Reusable systems require:
When organizations analyze total impact, including energy, water, chemical use, logistics, and instrument repair, optimized single-use systems often compare very favorably. In some scenarios, they can reduce the overall environmental footprint of a procedure while still improving safety and efficiency.
Modern single-use instruments benefit from advances in materials science and design. At ECA Medical, we:
These design choices help reduce weight, waste volume, and shipping impact. When paired with thoughtful hospital recycling and waste segregation programs, single-use instruments can play a part in a broader sustainability strategy that looks at the entire lifecycle, not just a single moment at the end of a case.
Shifting from reusable to single-use instruments is a strategic decision. It involves surgeons, administrators, supply chain leaders, and sterile processing teams. ECA Medical supports that transition with engineering depth, clinical understanding, and a long history in the field.
For more than 46 years, ECA has partnered with leading OEM implant companies and healthcare providers. We focus on precision torque limiters, sterile pack kits, and procedure-ready single-use Surgery-Ready™ instruments that serve trauma, extremities, sports medicine, joint reconstruction, and spine fixation and more.
We have shipped more than 53 million single-use instruments and hundreds of thousands of Surgery Ready kits worldwide. Each design reflects careful attention to ergonomics, tactile feedback, and OR workflow, so that when a team opens a kit, it supports their technique rather than forcing them to adjust to unfamiliar tools.
Developing a single-use solution involves multiple steps, from concept and design to testing, validation, manufacturing, and packaging. ECA brings these elements together in an integrated process.
We support partners by:
This integrated pathway reduces time to market and helps control cost, while giving OEMs and providers confidence that their single-use solution meets clinical, operational, and regulatory expectations.
Torque-limiting technology has always sat at the core of ECA’s value. Our TruTORQ and TruPWR systems deliver accurate, repeatable fixation while reducing surgeon fatigue, even in long or complex procedures.
By standardizing around these platforms, hospitals and ASCs can:
This consistency helps teams standardize outcomes and maintain a high level of quality across the entire network.
The direction of surgery is clear. Health systems want safer, more predictable workflows, and they need solutions that work within real-world staffing and budget constraints. Single-use surgical instruments and Surgery Ready kits address these needs directly.
Patient safety, workflow efficiency, and cost control no longer sit in separate conversations. They converge around practical tools that remove variability and support every member of the surgical team.
The first step rarely involves changing everything at once. Instead, hospitals usually begin with focused opportunities where single-use can deliver immediate value.
A typical approach might include:
Through this collaborative process, hospitals and ASCs can move into a future where instruments support the level of care patients expect, without overburdening teams or systems.
At ECA Medical, we remain committed to helping healthcare leaders build smarter, more consistent surgical workflows. With single-use Surgery-Ready™ solutions, we deliver precision, safety, and peace of mind in every case, one procedure and one patient at a time.