
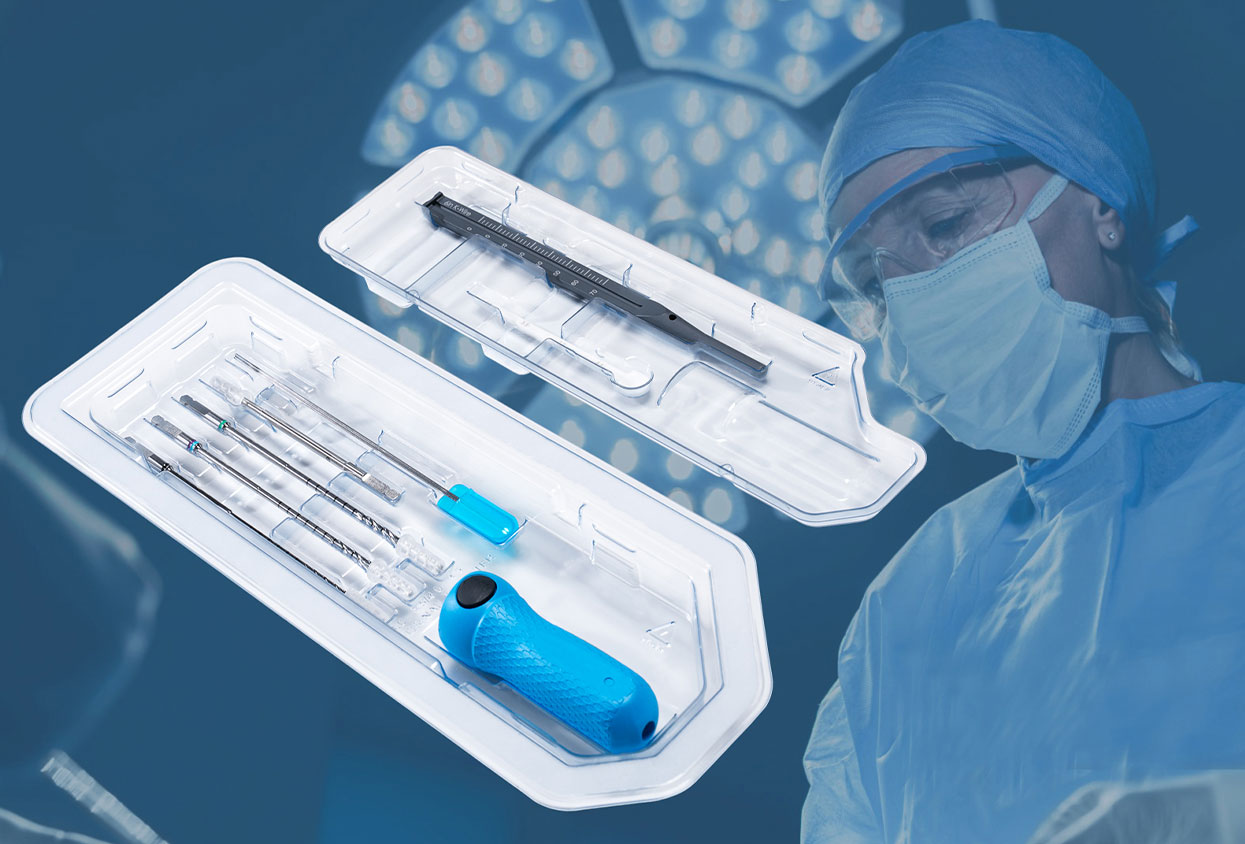
Complex procedures ask a lot of the operating room. Visibility can be limited, anatomy is not always forgiving, and decisions must be executed with accuracy the first time. When the field is deep or the construct is demanding, small variations in torque, grip, or reach become big differences in outcomes. That is why more clinical teams are reevaluating the tools in their hands, not only for performance but for consistency, case after case, often standardizing on Single Use Surgical Instruments to lock in sterility and repeatable control.
Over the last few decades, single-use surgical instruments have found their place in high-precision operations. What began as a practical way to reduce reprocessing risk now stands as a direct path to repeatable results. A calibrated driver that hits the same torque every time, a handle that fits the surgeon’s hand even at hour three, a kit that opens sterile and ready without missing parts, is now possible for every procedure.
The goal of this guide is simple. Help you choose single-use instruments that deliver confidence, sterility, and performance in high-risk, multistep procedures. If your work happens in trauma, sports medicine, joint reconstruction, or spine, you will find selection criteria, procedure-specific tips, and practical guidance for a kit that supports precision rather than getting in the way of it.
Reusable systems have served surgery for decades; yet everyone who has stood in a busy, sterile processing department knows their weak points. Torque delivery can drift as instruments age. Sterilization can fail for reasons that have nothing to do with intent. Wear shows up as slop in a driver or play in a handle at the worst possible time.The more complex the geometry, the harder it is to truly clean every surface. The faster the turnover, the more likely it is that a step is rushed or repeated.
Modern single-use instruments address those pain points head-on. They arrive factory-calibrated for the target torque, then locked and protected from the environment. Sterility is assured because the device is never reprocessed between cases. Materials and ergonomics are tuned to the specific procedure, which means the tool supports the surgeon’s technique rather than forcing a compromise. The result is not only a cleaner workflow but a tighter surgical performance envelope.
ECA Medical has spent more than 46 years building that envelope. Our surgery-ready™ single-use solutions were designed for precision first, then scaled for reliability. If you have heard of TruTORQ™ or TruPWR™, you already know the emphasis we place on calibrated control and surgeon comfort. What follows is a practical way to evaluate tools like these and match them to your procedures.
When you pick a single-use surgical instrument, you’re not just buying a tool; you’re buying certainty. In a busy OR or ASC, there’s no time to second-guess a torque setting, fight a sticky pouch, or discover a driver doesn’t mate with the implant on your back table. The right choice clicks at the exact torque it claims, feels steady in a gloved hand three hours into a case, opens cleanly without contaminating the field, and carries labeling your compliance team can stand behind.
This section focuses on the practical checks that matter most: verified torque accuracy (manual and powered),ergonomics that reduce fatigue, clean compatibility with your implant system (AO or ¼-inch square and system-specific tips), sterile-barrier validation and real shelf life, plus the boring-but-vital pieces, UDI, MDR/FDA documentation, and traceable lot numbers. We’ll also call out packaging behavior, kit layout, and inventory signals that keep turnovers smooth. In short, what to look for so the instrument does its job every single time, and your team can stay locked on the patient, not the hardware.
Fixation lives and dies on torque.Under-torque can lead to loosening. Over-torque can compromise bone or implant integrity. A single-use torque-limiting driver that holds a validated set point removes guesswork in the heat of a complex case. Look for instruments that are calibrated at manufacture, protected during shipping, and verified across the full labeled range. ECA’s TruTORQ™ portfolio is built around this principle so that a five newton-meter setting behaves like five newton-meters every time. When cases run long or access is awkward, powered options like TruPWR™ maintain torque targets without asking the surgeon to trade precision for speed.
Complex procedures are not sprints. Handle geometry influences fatigue, control, and the ability to apply a steady force along the correct axis. Evaluate T-handles for leverage in high-torque tasks. Consider palm and axial handles for deep access and fine control. Do not overlook offset patterns when anatomy or hardware placement demands a change in wrist angle to stay aligned with the pilot trajectory. The right grip and texture improve feel through gloves, especially when fluids make surfaces slick. In practice, ergonomics turns into straighter trajectories, cleaner seating, and less micro-correction.
Precision depends on interface integrity. Drivers must mate cleanly with the fasteners and connectors specified by the implant system. Confirm drive types and sizes, whether that is AO coupling, 1/4 inch square, or system-specific patterns. If your service lines cross multiple OEMs, consider dedicated kits per line to avoid mixing look-alike parts. A good rule: the fewer adaptors required, the fewer chances for play or misalignment. Purpose-built single-use kits from ECA are configured around the implant system, so instruments and implants behave as one construct rather than strangers inthe field.
Single-use only pays off if sterility is proven and preserved. Review the sterile barrier system, validation method, and real-world shelf life under your storage conditions. A kit that remains ready on the shelf for years without risk will support multi site inventory strategies and reduce last-minute scrambles. Look for packaging that opens cleanly on the sterile field, resists puncture on the way to the room, and carries clear indicators that confirm integrity at a glance.
Documentation matters more when you scale across sites or regions. Confirm that labeling and technical files meet FDA,ISO, and MDR expectations as applicable. Ask how lot traceability is managed and how complaints or field actions would be handled across an ASC network.When compliance is built in from the start, procurement gains confidence and clinicians gain time, because answers are already in the box.
Trauma is unforgiving of time and access.You want instruments that go from package to field without assembly, carry the torque you need for fracture fixation, and give control even when the angle is not perfect. A single-use trauma kit should prioritize immediate readiness and straightforward instrument layouts. In practice, that looks like preset torque drivers with tactile feedback, long axial handles for deep corridors, and depth gauges that read true without calibration steps. ECA trauma kits are organized so the next tool is exactly where the scrub expects it, which preserves rhythm when pressure is high.
Arthroscopic work and soft tissue fixation favor simplicity. Clean torque delivery is essential for anchors to seat reliably; yet the instrumentation load must stay light so the room does not drown in gear. Single-use drivers and ancillary tools designed around specific anchor systems reduce clutter and make count reconciliation easier.For shoulder or hip work where angles get extreme, ergonomic handles that allow a steady push through portals help anchors find home without stripping. The benefit is precision with less noise, both literally and figuratively.
Spine asks for deep access, stable axial control, and torque targets that span delicate and demanding steps. You may be seating a small set screw one minute and driving a higher-torque construct the next. This is where powered assistance like TruPWR™ earns its keep, reducing fatigue during long fusions while holding torque inside the validated window. Handle geometry matters because a small wobble at the surface becomes a big deviation at depth. Choose drivers with grips that keep the wrist aligned with the target trajectory even when retractors narrow the lane.
Hip and knee reconstruction present high-torque demands and complex sequencing. The kit should streamline setup and eliminate hunts for the correct driver, reamer interface, or depth gauge. A surgery-ready set with clearly labeled instruments and calibrated torque drivers supports reproducible seating of screws and locking elements. When every step lands where it should, the construct is stable, and the surgeon can spend attention on alignment and balance rather than on the tool.
There is no one answer for every service line. Off-the-shelf, pre-validated kits shine when you want fast deployment for common procedures, when your implants map cleanly to standard interfaces, and when you are proving value in a pilot. Custom kits make sense when you use proprietary implants, when brand-specific instrumentation is part of your market identity, or when you want the fewest instruments that can reliably complete your exact technique.
ECA Medical supports both paths. Rapid design cycles help you move from concept to clinical use without losing time to iteration. End-to-end validation means packaging, sterility, and performance are proven before your first case. Global manufacturing and logistics support keep kits flowing to hospital systems, IDNs, and ASCs, so a successful program scales without surprise.
“Surgery-ready” is not a slogan. It is a practical definition. Open the package, present the instrument, complete the step, and dispose per protocol. No assembly in the room. No maintenance. No recalibration. The gain is not only minutes saved. It is fewer opportunities for error and a calmer tempo for the team.
Setup time tends to shrink because the kit layout mirrors the procedure. Turnover picks up because there is no trip to central sterilization. In multisite systems, predictability improves because every room receives the same validated set rather than a local variation. Scrub techs learn one layout and apply it across the network. Procurement sees usage with clarity because each case consumes an identified bill of materials. Precision improves because the team’s cognitive load is lower, the tools behave the same way every time, and the field stays organized.
Sustainability is a legitimate concern, and it should be addressed with lifecycle facts rather than assumptions. Reprocessing consumes water, energy, detergents, wraps, and labor, and failed loads force repeats. When you step back and measure for a year, single-use can deliver a smaller footprint for selected procedures, particularly when the alternative requires complex trays and multiple cleaning cycles. Add the clinical impact, fewer repeat surgeries tied to failed fixation or contamination events, and the lifecycle picture improves again.
Waste streams are changing, too. Kits designed with minimal materials and recyclable components fit into hospital sustainability programs more easily than older designs. Compliance gains come along for the ride. When sterility is validated in the factory and chain-of-custody is documented to the field, survey answers get shorter, and risk managers rest easier.
One common mistake is ignoring the torque range your cases actually require. A driver that caps out below your peak need will tempt bad technique. Another is choosing solely on price. The low bid can be expensive when a driver does not mate cleanly with your fasteners, or when a handle geometry causes drift at depth. Teams sometimes overlook regulatory certifications and the quality of the technical file, which matters a great deal when you scale or cross borders. Buying without planning for OR integration leads to kits that sit on shelves because they do not match the way your rooms run. The last mistake is going it alone. Working with an OEM-aligned partner like ECA accelerates design, validation, and adoption because the path has been walked many times before.
Trust grows with repetition and results.ECA Medical has shipped more than 53 million single-use instruments and delivered over 300,000 validated procedural kits across spine, trauma, joint, and sports applications. That volume is not a vanity metric; it is how calibration, sterility, and ergonomics became reliable enough to bet cases on them.
TruTORQ™ torque-limiting drivers are built for repeatable targets with tactile feedback that tells the hand what the instrument is doing. TruPWR™ provides powered assistance without giving up control, which reduces fatigue in long cases and keeps torque inside the validated window. Behind those products sits a proprietary design and validation process that includes human-factors review, packaging engineering, and lot-level traceability. For implant OEMs, collaborative design programs and turnkey kit development shorten timelines and protect brand standards. For hospitals and ASCs, off-the-shelf options make it easy to start, and custom builds make it easy to fit.
If your next step is to explore what single-use surgical Instruments can do for your service lines, there are two straightforward paths. You can evaluate off-the-shelf, surgery-ready kits that map to common procedures, or you can co-develop a validated custom kit around your implants and technique. In both cases, you get instruments that are calibrated, sterile, and organized to support the way your rooms actually run.In both cases, the measure of success is the same: cleaner cases, steadier torque, fewer delays, and teams that can focus attention where it belongs.
For more than 46 years, we have helped surgeons, OEMs, and health systems move from good intentions to reliable performance with single-use, surgery-ready instruments. Our kits are designed to enhance precision in the procedures that challenge it most: trauma, sports medicine, joint reconstruction, and spine. We bring engineering, regulatory, and manufacturing under one roof so that ideas become validated tools, then validated tools become dependable programs across sites and regions.
If you want to reduce variability, simplify your workflow, and raise the floor on precision, ECA’s single-use Surgery-Ready™instruments are a smart next step, and we’d like to help. Explore our off-the-shelf solutions, or schedule a discovery call to map a custom kit for your system. Visit ECA to learn more, and let’s build a precision-first program that makes complex procedures feel more controlled, more consistent, and more efficient for everyone in the room, especially the patient.