
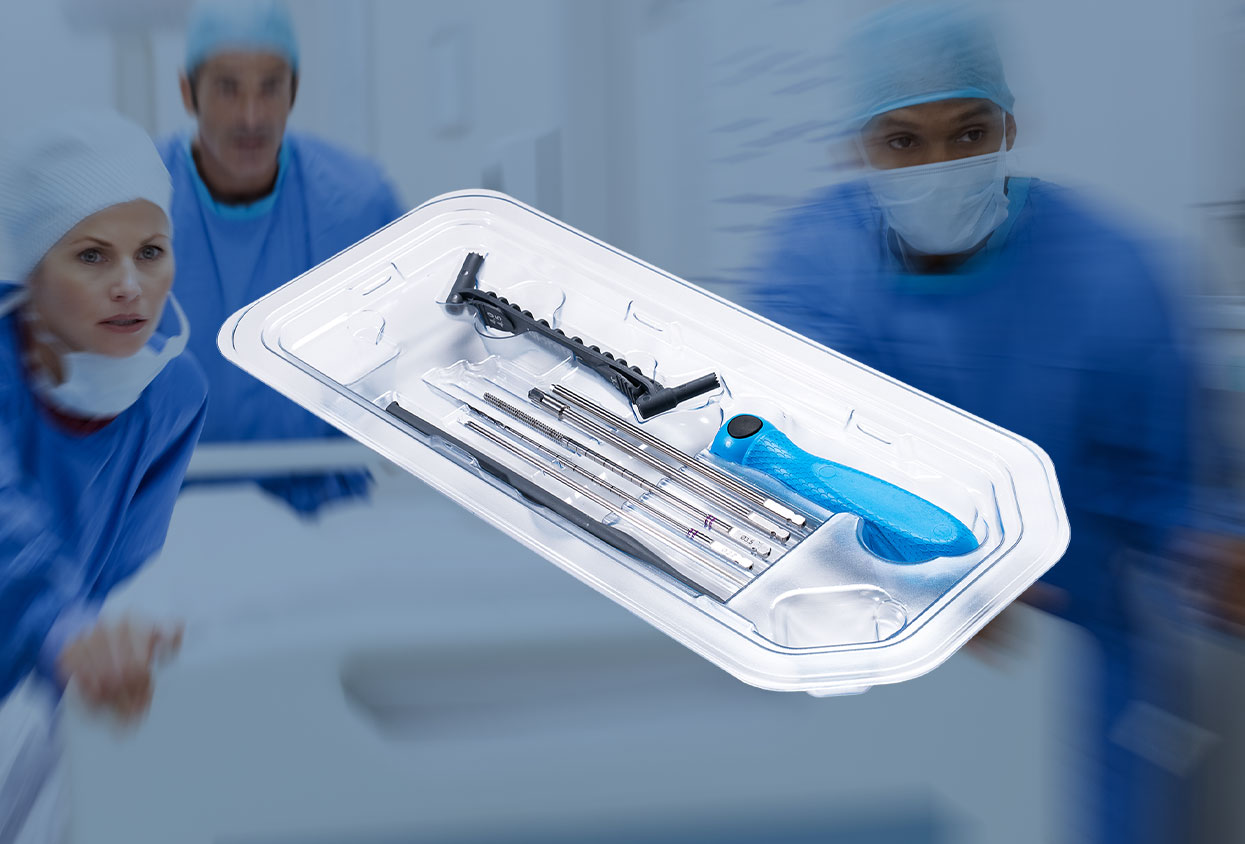
The bar keeps rising on hospitals and ambulatory surgical center efficiency. Patients want shorter wait times and smoother experiences; payers expect predictable value; and clinical teams are asked to do more with the same number of rooms. That pressure is most visible in high-volume specialties, where single-use surgical Instruments help keep schedules predictable: cases start on time, instruments perform reliably, and the turnover plan holds.
When we talk about high volume, we are including procedures such as orthopedic trauma where add-on cases can stack quickly, joint reconstruction where hip and knee procedures dominate the board, ASC-based sports medicine where anchors and soft tissue reconstructions run back-to-back, and spine programs where long, complex cases must stay on schedule. In all of these, the tools in the surgeon’s hand shape the tempo of the room. If the driver seats a screw at the right torque every time, the step vanishes into muscle memory. If the kit opens sterile and complete without assembly, setup becomes a quiet, repeatable routine rather than a scramble.
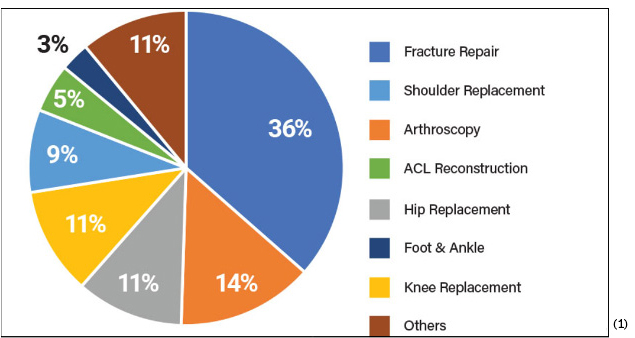
ASCs’ priorities and limitations differ from hospitals. Cost pressures are more acute as reimbursement rates are typically lower. ASCs also place a premium on efficiency, requiring devices that enable quick procedures and fast patient recovery. Additionally, storage space is often limited, necessitating compact equipment and streamlined inventory management. This all means the “full-system” options that were typically offered to hospital purchasing managers are often not right-sized for the ASC.(2)
This is why single-use surgical instruments are gaining ground. By arriving pre-sterilized, validated, and configured for the procedure, they remove common failure points that slow rooms down. ECA Medical has been and is one of the leaders in this shift, delivering surgery-ready, single-use kits that combine calibrated control, ergonomic designs, and clean packaging so teams can move with confidence from incision to closure.
Reusable instrumentation built modern surgery; yet its weaknesses show most clearly in fast-paced environments. Reprocessing takes time, attention, and resources, and those are exactly the things in short supply when the schedule is full. There will be evenings when sterile processing is working through a mountain of trays, mornings when the wrap on a critical set fails, or moments when a tiny component goes missing between the decontamination sink and the case cart. None of this is planned, but it is the real world of heavy volume and human limits.
Time-consuming reprocessing is the first bottleneck. Every tray requires the full cycle of cleaning, inspection, assembly, wrapping, sterilization, cooling, and documentation. Multiply that by the number of rooms, and you can see why a late add-on causes friction. Then there are sterile tray shortages that stem from maintenance downtime or unexpected demand, which show up as delays at exactly the wrong moment. Staff bottlenecks are common, particularly at shift changes or on weekends, and they increase variability in how consistently steps are executed. Delays also arise from maintenance or calibration needs, especially for torque instruments and moving mechanisms that have seen heavy use.
Each of these small cracks widens when volume climbs. A rushed step or a missed inspection introduces inconsistency into a process that must be consistent by design. The clinical risk is obvious, and so is the operational one. Delays increase OR costs because fixed resources are held idle, and patient satisfaction drops because schedules slip. The question for many leaders is not whether to work harder; it is how to design a system that asks less of people and more of the tools.
Surgery-ready™ and single-use are practical terms. A surgery-ready instrument is pre-sterilized, validated, and packaged so the scrub tech can open it on the field and present it immediately. Single use means one patient and one procedure, no reprocessing or cross-case handling, and no maintenance required after the final step.
When you put those together, the advantages are straightforward. There is no reprocessing cycle, so a case does not depend on the status of the washer or the availability of a wrap. There is no tray assembly or teardown, so turnover is less complex. Inventory becomes predictable because each kit maps to a specific procedure and lot, which makes readiness visible to the supply chain. Instrument counts are optimized to the task rather than inflated to cover many possibilities, which reduces clutter and speeds up counting in and out. The upshot is shorter setup, fewer last-minute scrambles, and staffing that can be planned around care rather than around equipment recovery.
In a high-volume context, those gains compound across a day. Ten minutes saved in setup translates to an earlier start for the next case, which preserves the slot after that and keeps patients moving through the system. Twenty minutes not spent hunting a replacement tool translates to a calmer room and a happier surgeon. These are not abstractions; they are the daily choices that make or break throughput.
Trauma surgeries thrive on immediacy. A fracture fixation case added at 3 p.m. needs instruments that are ready at 3p.m., not at 3:45 after a tray finishes cooling. Portable Surgery-Ready™ single-use sterile kits make that possible, whether the case is in a main OR or a satellite location. When distal radius plates or humeral fractures come through the door, the team can open a validated kit, find a calibrated driver with the correct interface, and proceed with confidence. The value here is not only speed, it is reliable control under pressure.
Sports medicine procedures are usually short, but they turn over rapidly in ASCs. That rhythm rewards streamlined kits built around anchor placement and soft tissue reconstruction. If a kit includes the exact driver interfaces for the selected anchors, a comfortable handle for portal work, and a depth gauge that reads true, the surgeon can complete the sequence without interruption. Because these cases run back-to-back, the benefits of single-use tools multiply. There is no waiting for reprocessing between patients, and there is less risk that a small part goes missing in the rush.
Spine surgery presents a different set of demands. Access is deep, precision must be maintained over a long period, and fatigue can introduce errors. Torque-limiting drivers for screws and rod constructs allow the surgeon to feel a consistent endpoint rather than guessing under glove and retractor pressure. Powered options reduce strain during long constructs while holding torque inside a validated range. In practice, this leads to more consistent seating, fewer stripped interfaces, and steadier hands late in the case.
Joint reconstruction benefits from Surgery-Ready™ kits as well. Hip and knee cases rely on a sequence of high-torque tasks that must be done right the first time. Single-use kits that include clearly labeled, calibrated drivers and procedure-matched accessories keep the setup clean and the steps predictable. When there is no downtime caused by a sterilization backlog or a missing insert, the room preserves the schedule, and the team preserves its attention for alignment and balance rather than logistics.
ECAMedical’s TruTORQ® and TruPWR™ instruments were designed for these realities. TruTORQ provides tactile, repeatable torque endpoints that build trust in the moment the fastener seats. TruPWR adds powered assistance without sacrificing control, which supports consistency during long or demanding steps.Both fit naturally into sterile pack kits that are organized in the order the work gets done.
Consider a busy ASC that performs anterior cruciate ligament reconstructions as a core service line. Twelve ACL repairs are scheduled most days, with the occasional add-on. Before moving to single-use kits, the center relied on reusable sets that required careful coordination with central sterilization. Turnover often stalled waiting for a tray to clear, and a missing driver tip might appear only after the patient was anesthetized. Each stall added minutes, sometimes more, and the last case of the day routinely started late.
After a pilot with ECA kits, the center shifted the service line to surgery-ready™ instruments for anchor placement and fixation. Setup time dropped because the kits opened in the order the surgeon worked. Turnover improved because no one waited for a washer cycle or a wrap to cool. Staff fatigue eased because the logistics workload was lighter. Over the first month, the center measured an average of eight to twelve minutes saved per case on setup and instrument handling alone, which translated into one earlier finish three days a week. Instrument loss fell sharply because the kit was the inventory unit, not a handful of loose parts. The infection-prevention team reported cleaner audit trails since every instrument opened had a traceable lot and a clear disposal path. For patients, the biggest difference was invisible; cases started on time and ended on time.
Numbers will vary by facility, but the pattern holds. When you replace a variable process with a validated kit, time and attention are returned to clinical work. When you remove reprocessing steps, you reduce the chance that throughput pressure harms consistency. That is the heart of efficiency in high-volume settings, precision in the steps that are repeated most often.
It’s easy to see the operational gains that faster OR turnover provides because it keeps the schedule intact and reduces overtime and more. Inventory becomes more predictable because each procedure consumes a known kit rather than an unpredictable mix of tray components. Reprocessing costs are eliminated for those instruments, which means fewer hours at the sink and fewer cycles through the autoclave. Scheduling reliability improves because rooms are not held waiting for tools, and maintenance events on reusable sets no longer ripple through a day of cases.
Staff satisfaction is another gain. Scrub techs work from kits that match the procedure, not from trays that try to be all things to all techniques. Nurses spend less time chasing physical assets and more time with patients. Sterile processing teams see a change in workload that gives them breathing room to focus on complex sets that remain. The perception of control increases across the board, which shows up in calmer briefings and more consistent handoffs.
Implant OEMs gain as well. Offering single-use instrument kits as a branded extension of an implant system creates a complete solution for surgeons and facilities. It reduces barriers to adoption in ASCs that are resource-constrained, and it sets a standard of performance that protects the implant’s reputation. In competitive markets, a clean, validated kit can be the difference between winning a service line and watching a competitor do it.
ECA Medical builds Surgery-Ready™ with high-volume realities in mind. The design model is collaborative, starting with the procedure and the implant system, then translating that into a kit that supports the actual sequence of steps in the room. The focus is on built-in torque control, ergonomics that stay comfortable when a case runs long, and procedural logic that puts each instrument where the hand expects it. Validation covers sterility, packaging integrity, and performance so that the kit is not only convenient, but it is dependable.
Global manufacturing and logistics ensure that kits arrive where they need to be, when they need to be there, across multi-site hospital systems and ASC networks. That capability is backed by a record that matters in healthcare, over 53 million single-use instruments shipped, and more than 300,000 surgery-ready™ kits delivered for spine, trauma, joint, and sports procedures. The trust of top implant OEMs worldwide did not happen by accident; it came from consistent delivery and attentive engineering.
Three questions come up in every executive review. Is single-use more expensive? Does it create more waste, and how do we switch without disrupting the service line? The cost answer starts with lifecycle math. Reusable instruments carry cleaning, assembly, wrapping, sterilization, cooling, storage, transport, maintenance, and repair costs, and they occasionally create delay costs when a set is not ready. Single-use kits eliminate those line items. When you add infection control benefits and labor saved, most programs find that validated single-use surgery-ready™ solutions deliver a positive return in high-volume use cases.
Waste is a real concern, and it deserves lifecycle thinking rather than an assumption. Reprocessing consumes water and energy repeatedly, and failed loads force repeat cycles. Packaging for large trays is bulky, and wrap failures generate additional waste. Modern single-use kits are designed with leaner materials and right-sized packaging. Some components can enter established recycling streams, subject to local rules. Combine that with fewer repeat procedures driven by instrument inconsistency, and the lifecycle footprint is often smaller than expected when measured across a year.
Transition planning is the final hurdle. The simplest path is to start with high-volume, high-risk procedures where the benefits are obvious; then run a dual-kit pilot so the clinical team can experience the differences in setup and flow. Education covers kit storage, opening technique, lot traceability, and disposal. Most facilities discover that adoption accelerates once teams feel the calmer tempo that the proper Surgery-Ready™ kit creates.
Standardization is how large systems create reliability. Hospitals and ASCs standardize on ECA when they want precision-engineered, torque-limited tools such as TruTORQ® and TruPWR™, when they value both off-the-shelf speed and custom design flexibility, and when they want faster room readiness with lower infection risk. Some choose ECA because the company can tailor solutions to different procedural volumes and implant platforms without forcing a one-size approach. Others choose ECA because they want a partner that can support an OEM collaboration, a hospital pilot, and a national rollout with the same level of focus.
Underneath those reasons is a simple test. Do the instruments perform exactly as labeled, case after case, and do they make life easier for the people who use them? When a kit answers yes, the organization sees fewer delays, steadier outcomes, and a more predictable day.
If you lead an implant platform, manage an ASC network, or run a hospital service line that lives in the high-volume world, single-use instruments are one of the clearest levers you can pull to improve efficiency without sacrificing safety. ECA Medical can meet you where you are. Explore off-the-shelf, surgery-ready™ kits that align with common procedures, or co-develop a validated custom kit that matches your implant system and your technique. The goal is not novelty; it is predictability, cleaner cases, steadier torque, faster turnovers, and teams that spend their energy on patients rather than on logistics.
For more than 46 years, ECA Medical has delivered sterile packs and validated single-use surgical Instruments and procedure kits for the cases that demand the most, from trauma and sports medicine to joint reconstruction and spine. The company’s track record includes over 53 million instruments shipped and more than 300,000 surgery-ready™ kits delivered, supported by collaborative design programs, end-to-end validation, and global manufacturing and distribution.
If you are ready to increase efficiency, reduce surgical backlog, and lower infection risks while maximizing performance, we would like to help. Reach out to learn how ECA’s single-use surgery-ready™instrument kits can support your goals. Together, we can build a high-volume program that runs on speed, safety, and scale, the three qualities that define a great surgical day.
1. https://www.grandviewresearch.com/industry-analysis/us-orthopedic-ambulatory-surgery-center-market-report
2. Orthopedic Design and Technology magazine, Sept. 23,2024. The Growth of ASCs for Orthopedic Procedures The growth of orthopedic procedures in ASCs represents a significant opportunity for medical device companies. Ilsa We beck