
Walk into any operating room today, and you can feel two pressures at once. First, the constant push to lower healthcare-associated infections. Second, an equally real need to make surgery safer, faster, and more sustainable. Leaders in perioperative care are increasingly turning to the Single-Use Instrument as part of a broader strategy to do both: prepare for surgery and protect patients.
ECA Medical is at the center of this change. We have supplied surgery-ready, sterile instruments and kits designed to improve infection control and simplify OR workflow. The focus is simple. Consistency, sterility, and speed without asking staff to do more with less.
Every hospital chases the same target. Fewer healthcare-associated infections, fewer surgical-site infections, fewer returns to the OR. The risk is not abstract. It shows up as a longer stay for a patient who should have gone home, a difficult conversation with a family, and a case that starts late because a tray did not clear quality checks. Anyone who has spent time near the sterile processing department at the end of a busy day knows how quickly small problems pile up. Carts arrive in waves. Decontamination sinks run nonstop and the autoclave queue grows three loads deep.
Reusable instruments built modern surgery. They also have weak spots that become obvious under pressure. Cleaning and inspection are meticulous tasks performed by people who are managing volume, shift changes, and the occasional missing part. A wrap can tear during transport. A count sheet can be wrong by one. A tiny O-ring can disappear down a drain. None of that means the process is careless. It means the process is difficult when timing gets tight.
Three failure patterns repeat in almost every facility. First is incomplete sterilization because the upstream cleaning step was less than perfect. Soil that hides in a hinge, a lumen, or a threaded recess can survive detergents and make sterilization less effective. Second is biofilm, the stubborn layer that clings to surfaces and resists removal. Third is geometry. Cannulated tools and complex assemblies challenge even the best technicians. You cannot reliably clean what you cannot see without time, technique, and the right brushes and adapters. When case counts climb or an emergency case is wheeled in, all of those risks grow because the system is trying to move faster than it was designed to move.
Even well-run programs have limits. A great SPD can keep trays flowing on a normal day and still struggle when two loaner sets arrive late or a washer goes out of service mid-shift. Validation steps can be correct on paper and still falter in practice if a load is packed too tightly or a peel pack is sealed a few millimeters shy of spec. Assembly errors are rare, but Infection-prevention teams can see the pattern. They know the people are capable and the procedures are sound; yet the combination of volume and variability creates gaps that are hard to close with training alone.
A more personal way to say it is this. If you have ever waited in pre-op with a patient while someone calls downstairs to check on a tray, you understand what “process risk” feels like in real time. Compliance, quality, and throughput are all pulling on the same rope. The fewer moving parts you rely on between cases, the less likely you are to trip over one of them.
Most changes in surgery follow the same arc. At first, there is resistance. Finance asks about cost. Sustainability asks about waste. Surgeons and techs prefer what they already trust. That is how single-use instruments entered the room, as a cautious experiment rather than a mandate.
The picture changed when high-volume services put them to work. Ambulatory surgical centers wanted predictable turnover. Orthopedic trauma needed instruments that were ready the moment a late fracture case hit the schedule. Sports medicine programs wanted anchor drivers that felt the same from the first case of the morning to the twelfth of the day. Those teams did small pilots and reported back simple, tangible wins. Cases started on time because there was no dependency on a washer cycle. Instruments felt consistent because torque and fit were set at manufacture, not influenced by age or maintenance. Count in and count out were faster because the kit was right-sized to the procedure. Surgical-site infection rates ticked down and the rooms felt calmer.
Then the pandemic arrived and turned an interesting option into a practical necessity. Infection control moved to the front of every discussion. Cross-contamination risk had to be eliminated wherever it could be. Staffing was stretched, loaners were delayed, and reprocessing backlogs showed up often. Hospitals and surgical centers that had been dabbling with single-use trial kits shifted quickly to targeted use by procedure type, then to broader adoption once they saw the operational relief. Supply chain leaders liked the traceability. Risk managers liked the audit trail. Surgeons liked that the instrument in their hand behaved the same way, case after case.
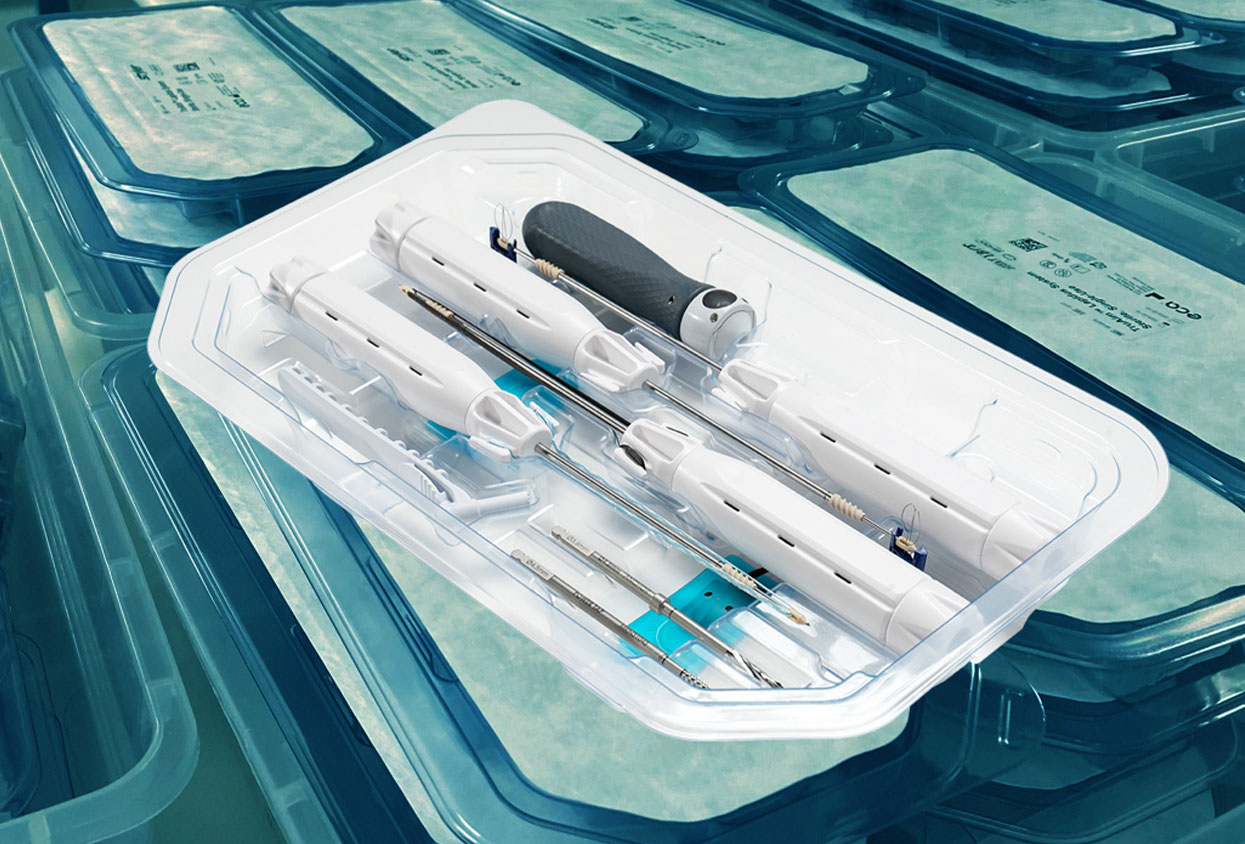
The instruments themselves matured at the same time. What began as a small set of basics expanded into a full family designed for real orthopedic work. You now see single-use torque-limiting drivers that give clear tactile feedback when you hit the set point. Depth gauges that read true without calibration steps. Pin and screw drivers with the correct interfaces for the implant system in the room. Cutters and ancillary tools that arrive sterile and organized in the order the case flows. In many programs, the kit is no longer a novelty. It is the default for the procedures where consistency and speed matter most.
If you’ve ever chased a missing driver at 7:02 a.m., you already know why infection-prevention personal like single-use. Their job is to count touchpoints and remove them. Every handoff, washer, assembly, wrap, sterilizer, cool-down, transport, peel, adds a place where a tiny hiccup can turn into a big problem. With a single-use kit, most of that chain disappears.
The routine gets simple: peel the pouch, present the instrument, use it, and discard it per protocol. No detours to SPD, no load to validate, no “did that seal look funny?” debate at the back table. Lot numbers and UDI are right on the packaging, so traceability stops being a scavenger hunt through stickers and logbooks. And because nothing returns for cleaning, there’s no lingering doubt about instrument sterility on a busy night.
Teams that switch targeted procedures to single-use describe two changes right away. First, fewer stalls. The kit opens, the tools work, and the case starts on time. Second, the room feels calmer. When the scrub isn’t improvising and the surgeon’s hand gets the same torque “click” every time, people breathe easier.
ECA Medical’s Role in Reducing Infection Risk
Consistency comes from repetition. ECA Medical has shipped more than 53 million single-use instruments and over 300,000 validated kits across spine, trauma, joint, and sports procedures. At that scale, the small things matter: pouches that open without showering the field, labels that scan on the first try under bright lights, paper that doesn’t glare, trays that don’t force a reach across the sterile line.
Our kits are surgery-ready. No assembly in the room. No recalibration between cases. Each component is validated, sealed, and tracked so the chain-of-custody reads cleanly from the manufacturing line to the Mayo stand. Torque-limiting tools, TruTORQ™ for manual work and TruPWR™ when power helps, are calibrated so a five newton-meter setting feels like five newton-meters, not “close enough.” If a surgeon has to pause and wonder whether a handle was cleaned well last night or whether a driver will hit its mark, we haven’t done our job. The point is to let the team spend attention on alignment, fixation, soft-tissue balance, everything that changes outcomes, not on whether the tool will behave.
Use Case Spotlight: High-Risk Procedures, Lowered Contamination
Late-day trauma in an ASC. It’s 4:10 p.m., the board just got a distal radius, and SPD’s autoclave queue is three loads deep. In the old flow, you’d be checking on a tray, cooling times, maybe borrowing a backup. With ECA’s single-use Surgery-Ready™ kits, the nurse pulls the sealed kits, the scrub opens in order, and the room rolls. No waiting for a cycle, no hunting for a tiny insert, no debate about a wrap that snagged on the cart. The contamination window narrows because there’s no reuse loop at all, one patient, one instrument, done.
Peak-season sports medicine. Twelve-case shoulder day. The procedures are short; the pressure point is turnover. Even a great SPD feels the drawdown when cases run back-to-back. Single-use anchor drivers and gauges arrive sterile and consistent, so the room resets at the pace the schedule expects. Counts are faster because the kit holds exactly what the technique needs, nothing extra to sort, nothing missing to chase. Save a few minutes on each case, and you protect the last slot from sliding into the evening.
Emergency surgery when minutes matter. A hip fracture rolls in, anesthesia is ready, and you need to move. Kits that eliminate assembly, cleaning, or calibration shave prep time and cut touchpoints before incision. Over a month, those saved minutes turn into real capacity. Over a year, the labor you’re no longer spending on brushing, wrapping, loading, cooling, and documenting flows back to clinical work. Hospitals that model the change usually see two lines move together: fewer opportunities for contamination, and thousands of staff minutes returned to the people in the room.
It is fair to ask about waste. Many of us did when single-use instruments first hit our committees. What changed the conversation was lifecycle math. Reprocessing is not free. It takes water, energy, detergents, sterilants, packaging, staff time, and equipment maintenance. When you add it up across a year, the environmental footprint of reuse exceeds the footprint of single-use for selected procedures.
The newer generation of single-use kits is also designed with leaner material mixes and right-sized packaging. No bulky trays. No wrap failures that force a re-sterilization cycle. No second trip through the washer because a lumened device failed inspection. Infection prevention and sustainability can align when you remove reprocessing loops.
Finance leaders want two things: fewer surprises and fewer readmissions. Single-use programs help on both counts. Infection reductions show up as avoided costs. Fewer revisions. Fewer extended stays. Fewer penalties are tied to quality metrics.
Sterile processing departments feel the relief, too. The backlog shrinks. Staff can focus on complex trays that truly need expert attention. Overtime drops because the evening surge is smaller. Inside the OR, there are fewer delays from missing instruments or failures that are discovered after the patient is already prepped.
Budgeting gets cleaner. There are no repair bills, no emergency rentals, and no calibration programs for torque instruments. Instead, there is a known per-case cost that maps directly to volume.
Hospitals choose ECA for the same reasons surgeons do. Kits are validated and ready. The torque-limiting drivers do what they claim to do, which is set and hold a target value case after case. That includes our TruTORQ™ and TruPWR™ families built for accuracy in orthopedic, trauma, and spine work.
The other reason is fit. We create tailored solutions for implant OEMs and hospital systems so that the kit matches the procedure rather than asking staff to adapt to a generic toolset. When a kit is designed around the actual steps of a case, the flow improves, the field is tidier, and the cognitive load on the team goes down.
Feedback we hear most often is short and to the point. “It just works.” Infection prevention appreciates the chain-of-custody detail. Surgeons appreciate the feel and the repeatability. SPD appreciates that there is one less complex tray in the pile.
The regulatory environment is not standing still. Guidance from public health bodies and accreditation standards keeps tightening around cleaning validation, documentation, and device design. In the European Union, the MDR framework increased expectations around traceability and usability. In the United States and elsewhere, site surveys look closely at reprocessing steps and provide proof that every stage is validated.
Many international systems have leaned into single-use for selected procedures as a straightforward way to comply. It is easier to prove sterility when each instrument arrives sterile, sealed, and tracked. ECA’s global footprint allows us to support deployment plans across regions with local regulatory alignment and custom validation data where needed.
Single-use is moving beyond orthopedics. You can already see growth in neuro, ENT, vascular, and general surgery, where precision and sterility are non-negotiable. The technology around the instruments is evolving as well. Expect smarter packaging, integrated track-and-trace, and digital audit trails that make it easier to show inspectors exactly what happened to each kit.
The future seems clear. We are moving toward ORs where prevention is built into the tools themselves. Not just cleaned better, but designed so that contamination risk is engineered out of the process.
ECA Medical has been building toward this future for more than 46 years. Our instruments are in use every 10 seconds somewhere in the world. That scale is not a vanity metric. It is proof that single-use can meet clinical expectations at volume.
We partner with implant OEMs and hospital systems in two ways. First, by converting reusable sets into validated, single-use kits that mirror the existing workflow. Second, by building new kits from the ground up, with engineering, regulatory, and manufacturing support handled under one roof. The result is a path to infection reduction without a compromise on performance.
If your job touches infection prevention, you know the steps: Hand hygiene, antibiotics, skin prep, airflow, device choice, and process reliability. Single-use instruments sit squarely in that last category. They remove variability where it hurts you most and give back time to focus on the patient.
ECA can help. Whether you are running a busy ASC, overseeing multiple hospitals inside an IDN, or building the next generation of orthopedic implants, ECA Medical can design a single-use kit that reduces infection risk, keeps OR prep consistent, and eliminates the friction of reprocessing. No assembly required. No recalibration. No second guesses.
If you want to see what this looks like for you, reach out for a consultation.