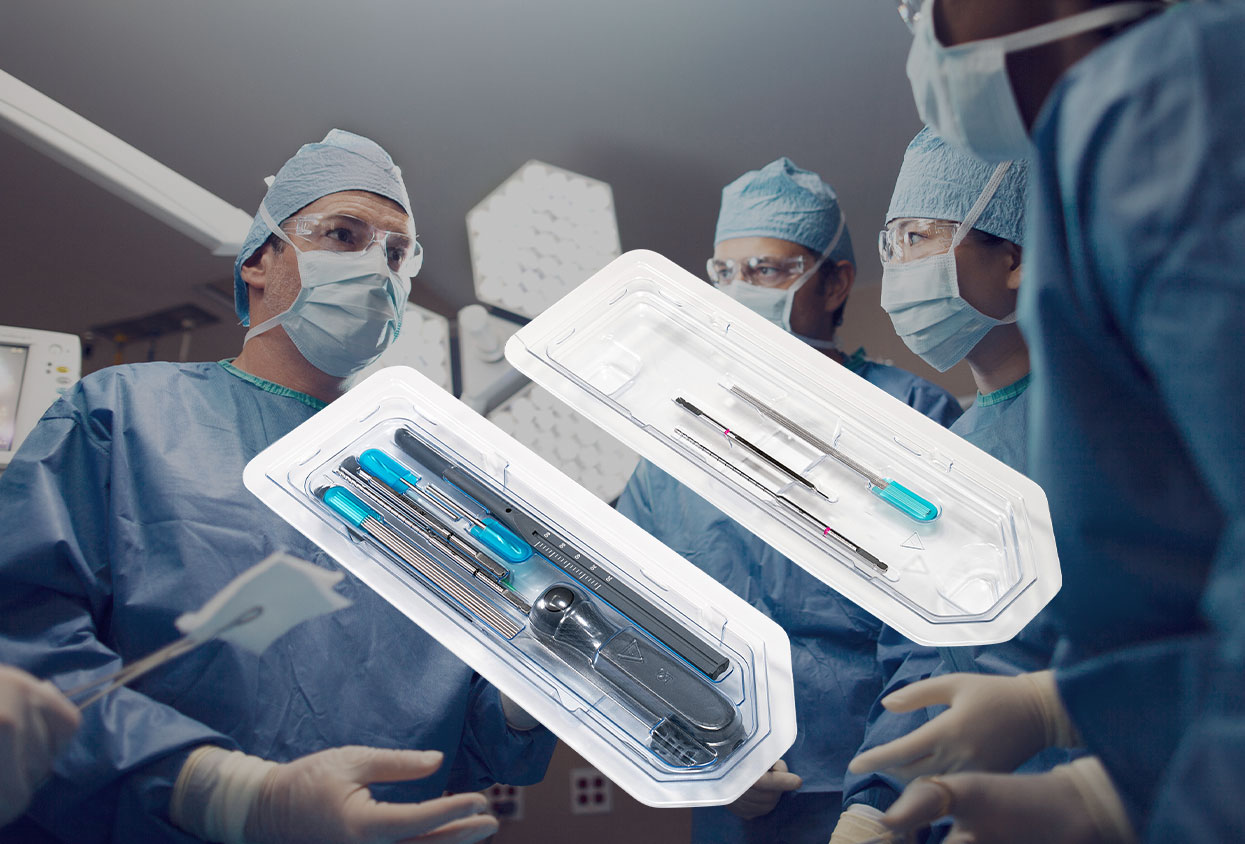
A New Era of Surgical Precision and Preparedness
Today’s hospitals are at the threshold of a transformation in the operating room—one driven by the need for true efficiency, increased safety, and reliability. Surgical teams face growing complexity: more patients, tighter schedules, and heightened expectations for precision. For ECA Medical, redefining the surgical workflow is not just about creating innovative products—it's about designing solutions that make surgery smarter, safer, and more sustainable.[1]
This post explores how Ready-to-Use Surgery Kits and advanced Sustainable Instrument Solutions are setting a new standard across healthcare. Through collaboration with OEM partners, ECA is bringing purpose-built kits and instruments to the front lines, directly addressing today’s logistical and clinical challenges.
Rethinking Surgical Workflows: Identifying the Pain Points
Traditional operating rooms often rely on reusable surgical tools and manual sterilization routines—an approach that brings friction and unpredictability:
- Sterile processing bottlenecks: Every reusable item must be meticulously cleaned, inspected, packaged, sterilized, and validated. In busy hospital settings, this can delay procedures and create stressful bottlenecks for sterile processing staff.
- Variable instrument quality: Instruments subjected to repeated use may lose their edge or alignment, causing performance drift and forcing surgeons to adapt mid-case.
- Risk of cross-contamination: Even strict protocols cannot fully eliminate residual biofilm or contaminants, increasing infection risk for patients.
- Complex logistics: Tracking large sets of instruments, replacements, repairs, and sterilizer cycles places a significant administrative burden on hospitals.
All these pain points contribute to unpredictable delays and greater stress for clinical teams—especially as the number and complexity of surgical cases continues to rise globally. Consistency and predictability are critical, and ready-to-use surgical kits help achieve both.
The Solutions: Ready-to-Use Surgery Kits Explained
What Are Ready-to-Use Surgery Kits?
Ready-to-Use Surgery Kits—often called Surgery-Ready™ kits—are fully assembled, pre-sterilized instrument packages custom-designed for each surgical case. They contain all tools, disposables, and specialty devices required to perform a procedure, optimized for both hospital and ambulatory surgery center environments.
How Do They Work?
- Case-specific design: ECA Medical and its OEM partners engineer each kit for a particular procedure, such as fracture fixation or joint replacement.[1]
- Sterilization and packaging: Every instrument is assembled under strict conditions, sterilized, and organized into labeled trays or modules for intuitive use by the surgical team.
- No hospital prep required: The kits arrive at the facility ready-to-open—meaning there is no need for additional sterilization or tray assembly prior to the procedure.
These benefits enable swift transitions in the OR, reducing preparation time and allowing clinical teams to focus on what matters: patient care.
Application Across Surgery Types
Ready-to-Use Surgery Kits are making a difference in multiple specialties:
- Orthopedic & spine surgery: Torque limiting instruments and fixation tools embedded in ortho kits enhance control and reliability for procedures involving screws and implants.
- Cardiovascular & implant procedures: Kits include torque drivers, precision clamps, and auxiliary instruments tailored for vascular work and device placement.
- Ambulatory Surgery Centers (ASCs) & hospitals: These facilities gain new efficiencies by using pre-packaged, pre-sterilized kits—lowering their dependence on internal sterilization infrastructure and enabling faster setup and turnover.
By removing the burdens of manual preparation and ensuring every tool is ready and calibrated, ECA Medical’s kits increase procedure speed and safety across the board.
How Ready-to-Use Kits Streamline Surgical Workflows
Efficiency in the operating room is achieved not just with better instruments, but with highly organized workflows:
- Rapid verification and deployment: Kits are organized so surgical teams can quickly identify and deploy all instruments without hunting for missing or mismatched parts.
- Consistent instrument quality: Instruments are new and unused for every case, eliminating variation in sharpness, calibration, and mechanical performance.
- Reduced stress and errors: Streamlined preparation means less stress for surgical teams and fewer opportunities for logistical errors—helping ensure procedures run on time.
This approach is especially critical as operating rooms see higher patient throughput and increasing demands for accountability and quality.
Enhancing Sterility and Safety with Single-Use Instrumentation
Sterility is non-negotiable in surgery. The reality is that, even with rigorous cleaning protocols, reusable instruments can still harbor contaminants. Single-use instruments, a core feature of ECA’s ready-to-use kits, directly address these risks:
- No cross-patient exposure: Each instrument is new and sterile, removing the danger of residual contaminants or biofilm.
- Minimized process steps: By eliminating hospital-based sterilizer cycles and manual cleaning, there are fewer chances for human error or incomplete sterilization.
- Uniform performance: Single-use guarantees every tool is at optimal sharpness, alignment, and function.
For clinical teams focused on safety, this translates to greater confidence in every procedure, and for patients, it means less risk and improved outcomes.
Sustainability in Surgical Innovation: Toward Responsible Use
Surgical kit innovation must balance patient safety with environmental responsibility. ECA Medical is at the forefront, designing Sustainable Instrument Solutions:
- Hospitals produce large volumes of regulated waste. Single-use instruments can contribute, but smart design can mitigate impact.
- Reusable instruments consume energy and water. Processing and maintaining reusables requires resources; in some studies, single-use kits show a lower carbon footprint depending on context and usage volume.
- Sustainable components: ECA Medical develops kits using recyclable materials and green packaging, reducing landfill and supporting material recovery initiatives.
- Efficient waste streams: The company participates in take-back programs, segmentation of regulated waste, and protocols that support environmental compliance.
ECA’s commitment: Engineer disposables with maximum performance and minimum ecological footprint, so hospitals can achieve sustainability goals without compromising safety.
Global Impact: Transforming Healthcare Systems at Scale
Ready-to-use kits and sustainable instruments are reshaping surgical practice worldwide:
- Lower procedural costs: Hospitals and ASCs that adopt ready-to-use kits experience predictable savings and simplified supply chains.
- Consistent clinical outcomes: Kits deliver consistent results, supporting data-driven improvement in patient outcomes.
- Streamlined logistics: Lower reliance on complex reprocessing infrastructure means fewer delays and more time for patient care.
- Value for OEMs: ECA Medical partners with OEMs to design kits around implants, elevating their products and creating turnkey solutions for clinics, hospitals, and surgery centers.
Whether in high-volume metropolitan hospitals or resource-limited settings, these innovations enable scalable, transparent workflows—making every procedure count.
Quality and Compliance: The Foundation of Trust
At ECA Medical, trust begins with rigorous adherence to quality and regulatory standards:
- ISO 13485 compliance: Every kit meets global device quality management standards and passes stringent FDA, CE/MDR, and worldwide health authority requirements.
- Sterilization, packaging, and validation: Instruments are subjected to robust sterilization validation, packaging integrity checks, and performance testing.
- Precision manufacturing and traceability: Torque limiting instruments, a specialty of ECA, undergo factory-calibrated testing within strict tolerances. Every kit is tracked by lot code, serial numbers, and QR codes for real-time audit or recall.
Quality checkpoints span the entire instrument lifecycle, from design and raw materials through post-market feedback—ensuring every product that arrives in the OR meets surgeons’ highest expectations.
Looking Ahead: The Future of Surgical Kits and Sustainable Instruments
Innovation never sleeps. ECA Medical is shaping the future of surgical workflow standardization:
- Automated kit assembly: Robotics may soon handle kit preparation, torque calibration, and final packaging, reducing human error.
- Intelligent traceability: Barcodes, RFID, and digital tracking systems empower surgical teams to verify kit contents and automatically document for hospital compliance.
- Recyclable, high-performance materials: Advanced bioplastics, compostable films, and metals optimized for recovery will shrink surgical waste footprints.
- Hybrid models: Some instruments may be returned to the manufacturer for responsible processing, allowing a blend of single-use convenience and sustainable reuse.
- Wider application: Ready-to-Use kits could become standard in specialties such as neurosurgery, urogynecology, and ENT.
These trends will spark further conversations about balancing surgeon readiness, patient safety, and sustainability.
Conclusion: Streamlined, Sustainable, and Surgery-Ready™
ECA Medical is the trusted choice for leading orthopedic implant OEMs and hospitals seeking proven innovation in surgical tools. With over 46 years of dedication to single-use instruments and procedural kit design, ECA Medical accelerates product development and streamlines surgeries while championing sustainability.
ECA’s team of clinical and engineering specialists partners with OEMs to create trauma, extremity, sports medicine, large joint, and spine kits that reach the market faster and perform reliably. Using advanced materials and precision engineering, ECA delivers high-performance, surgery-ready™ kits to hospitals, ASCs, and clinics everywhere.
Surgeons and operating room teams benefit from faster setup, more efficient workflows, and simpler cleanup, allowing them to handle more cases per day and provide better care. That’s the ECA difference—trusted by industry leaders, built for performance, and designed to advance healthcare one procedure at a time.
Discover how ECA Medical makes your next procedure smarter, safer, and more sustainable. Ready-to-Use Surgery Kits and Sustainable Instrument Solutions are not just the future—they are redefining the standards of care today.
Contact ECA at sales@ecamedical.com or (805) 376-2509 to learn how our team can tailor single-use procedural kits and instrument solutions for your needs.